"Good Heavens what insect can suck it?"
The stranger-than-fiction miracles of coevolution in nature and human society. (Or: what connects avocados, ants who farm fungus, karma, and our relationship with dogs?)
Thank you for reading The Garden of Forking Paths. This edition is for everyone, but consider upgrading for just $4/month to support my writing and research—so I can keep at it. I rely exclusively on your support. Paid subscribers get full access to the archive of 215+ essays. You can also support my work by buying my book, FLUKE, twice-named a “best book” of 2024.
I: The Moth With the Footlong Tongue
On January 25, 1862, Charles Darwin received a box containing several exotic specimens of elegant, beautiful orchid flowers. The orchids were a gift from James Bateman, accomplished horticulturist, debonair landowner, and, above all, devoted orchid enthusiast.1 But the flowers were not labeled, so Darwin wrote to Bateman for clarification.
A few days later, a letter followed, with a detailed list of the flowers that had been provided.2 The letter listed the six enclosed species, including the final one: Angraecum sesquipedale of Madagascar.
Darwin was instantly mesmerized by that orchid.3 Close inspection gave way to astonishment, then bafflement. Darwin particularly marveled at the nectary—the area that produces and stores nectar so that pollinators will be attracted into the flower. It was nearly twelve inches long, down a narrow, inaccessible tube, making it effectively impossible for any known species to get access to the sugary delights within. How could this Madagascan flower with a freakish, footlong nectar spur get pollinated and reproduce?
Presumably in a state of extreme intellectual agitation and excitement, pen shaking with scientific zest, Darwin fired off a letter to his close friend, J.D. Hooker, providing one of the better lines in the history of science4:
Good Heavens what insect can suck it?
Five days later, with this orchid still clearly on his mind, Darwin followed up with a firmer prediction to answer his own question: “What a proboscis [tongue] the moth that sucks it, must have! It is a very pretty case.”
There were no known moths with footlong tongues—and certainly nothing similar had been discovered in Madagascar. But Darwin’s deduction was straightforward: if the flower had evolved a nectary that was 11.5 inches long, then that could only be because it had evolved, in tandem, with a pollinator who now must also have a proboscis of the same length. Somewhere in Madagascar, Darwin predicted, this beautifully freakish flower had an evolutionary partner: a moth with a freakishly long tongue.
Darwin reported that his claim was ridiculed by entomologists, amused and bemused by the silly notion of a little moth flitting around with an absurdly unwieldy tongue protruding from its head.5
Then, in 1903, decades after Darwin’s death, a new moth species was discovered in southern Africa: X. morganii praedicta, the predicted moth. It was the strangest moth imaginable, but it fit Darwin’s description: its tongue, which neatly rolled up into its mouth, was far longer than its body. Behold!
However, nobody could verify for certain that this was the moth that pollinated Darwin’s favored orchid. Finally, in 2004, Philip J. DeVries of the University of New Orleans camped out in Madagascar overnight next to some orchids, used an infrared light, and got lucky: he captured video of the moth unfurling its tongue and sucking nectar out of the orchid’s footlong nectary.
After 142 years, Darwin’s peculiar proposition was fully vindicated.
Darwin’s Orchid and its corresponding moth is a textbook example of coevolution—a well-established and mind-blowing principle in biology, which offers a unique prism for understanding the natural world. This is the realm of ants who farm fungus; of newts who poison snakes with neurotoxins and snakes who eat them anyway; of evolved mind-control and of absurdly elaborate deception, mimicry, and innovation.
But, as we’ll soon explore, coevolution is also an underappreciated tool for better understanding human societies—and ourselves. With that evolutionary framework, we can reveal the complex inner workings of everything from allergies, dogs, and arms races to the rise of empires, cybercrime, and karma.
To understand those dynamics, though, we must first briefly explore how the process actually works.
II: The Scientific Magic of Coevolution
Biological coevolution refers to a reciprocal process of evolutionary change, in which changes in one species cause a corresponding adaptation in another. (Human-dog interactions over thousands of years are a prime example of coevolution, a fascinating dynamic that we’ll return to shortly.)
These adaptations happen because of shifting selection pressures. To illustrate this concept, consider how Darwin’s Orchid ended up with a footlong nectary. In every generation of orchids, there are slight variations in the length of the spur that leads to the nectar, some longer, some shorter. The problem for the orchid is that some opportunistic insects steal the nectar without providing any pollination.
With Darwin’s Orchid, flowers with shorter spurs were prone to having their nectar stolen by free-riding insects other than the pollinating moths, which hurt their ability to reproduce. By contrast, orchids with longer spurs would have fewer insect freeloaders. Even better, when the moths arrived on those flowers, they would have to press their proboscis deep into the nectary, just at the limits of their tongue’s reach. Pressed up against the flower like this, it was more likely that pollination would occur successfully.
Over time, this created a back-and-forth adaptation between flower and moth; orchids with longer nectaries staved off free-riding nectar thieves while amplifying successful pollination from the moths, while those with shorter nectaries were less likely to succeed in the evolutionary sweepstakes of reproduction. Moths with longer tongues could get nectar that was unavailable to other insects. Over time, this dynamic forged two very strange creatures, perfectly adapted to one another, in mutualistic coevolution.
Coevolution is a complex tinkering process, down to the level of a dizzying array of genes, but the key principles are easy to understand:
Coevolution depends on a change in one species that will affect the reproductive success of another. (If a change in one species lowers or increases the average likelihood that another species will survive long enough to successfully reproduce, that’s going to matter for coevolution.)
It also depends on the frequency of interaction between the species. (If they interact often, a coevolutionary relationship is far more likely.)
The dynamics of coevolution are altered by various forms of evolutionary potential, such as the length of generations; whether the species reproduces sexually or not; and how much genetic variation exists in the traits that are evolving.6
Coevolution doesn’t always happen evenly, so there can be hotspots and coldspots, even if you have the same species interacting—geography and ecology matters. (For example, certain newts produce Tetrodotoxin (TTX), an extremely potent neurotoxin that is significantly more lethal than cyanide. Some snakes that eat those newts have evolved resistance to TTX, which has pushed the newts to evolve more potent forms of the neurotoxin, in a vicious cycle. But in environments where newts have less potent neurotoxin, the snakes have less resistance to it, and so on.)7
Now, let’s consider the logical conclusion from the newt and snake example. If newts get stronger toxins and snakes evolve resistance to it, don’t they just end up in the same place as where they started? In other words, don’t they eventually settle into an evolutionary stalemate?
The answer, often, is yes—and that’s a core pillar of an influential idea in evolution known as The Red Queen Hypothesis (not to be confused with The Red Queen Fallacy in modern human society, a term which I coined and wrote about here):
The Red Queen Hypothesis came from Leigh Van Valen, an influential evolutionary biologist who argued extinction rates don’t fluctuate as much as one would think, instead remaining largely stable over longer time scales.8 The idea is simple, but profound: every species is constantly adapting to keep up with others, so when one gets stronger, that will trigger adaptations in other species, who will also evolve, in a non-stop evolutionary arms race.
Like the Red Queen in Through the Looking Glass, every species races to get ahead, but because every other species is also racing to get ahead, they’re all just racing frantically to avoid going extinct—and often ending up in the same place as where they started. This matters because it shows how constant, frantic adaptations might not yield any overall gains in the system, akin to an evolutionary treadmill without an off switch. And as we’ll see in a moment, this dynamic has implications for plenty of human social problems, too.
III: Ant Farmers, Devious Birds, Evolutionary Ghosts
Around sixty-six million years ago, in the aftermath of the asteroid that wiped out the dinosaurs, the toxic cloud of dust and gas also disrupted photosynthesis. This gave fungi a temporary edge over plants—and it also meant that small invertebrates who fed on little bits of food, particularly underground, were able to thrive while countless other species died out.9
It is around this time—and possibly even during this short window of a few months—that a species of ant may have begun to farm fungus as a sustainable food source. It was a textbook case of domestication of a crop; ants became farmers roughly 66 million years earlier than we did. They grow “fungus gardens,” tend them, then eat their harvest. (Research published in late 2024 in Science demonstrated that it’s likely that ant farming of fungus evolved not once but twice, since it was such an effective tool to help ants survive. Today, there are 240+ known species of ants that farm fungus.)
When a leafcutter ant queen starts a fresh colony, she carries a little bit of fungus in her mouth. She tends that initial fungus “seed” while waiting for workers to mature. When they do, one set of (larger) workers goes out foraging, gathering leaves to feed the fungus. Another set of (smaller) workers tends to the fungal garden, weeding it and licking it to keep it clean.
The farmed fungi have evolved a special growth, known as gongylidia, which is used to feed the ants. Over time, coevolution created such a tight bond between the fungus and the ants that some became “obligate mutualists,” which means that each cannot live without the other. If the ants died out, the fungus couldn’t survive, and vice versa. Evolutionary specialization has trapped them in a co-dependent relationship.
But the ants and farmed fungi also have a shared problem: a parasitic microfungus that can infect the colony and decimate the food supply. Those parasitic species have evolved alongside the ants and the fungus, in an adversarial arms race. And here’s where the science gets truly mind-boggling.
Many of the antibiotics we use come from Actinomycetota, or Actinobacteria.10 Within that group, one strain of bacteria is particularly effective at killing off the microfungus that infects the fungal gardens cultivated by the ants. Astonishingly, the ants have evolved changes in their exoskeleton “supporting their growth through glandular secretions” that ensure that helpful bacteria will grow on their bodies. It forms a sort of bacterial shield across their bodies, specifically to protect the fungus.
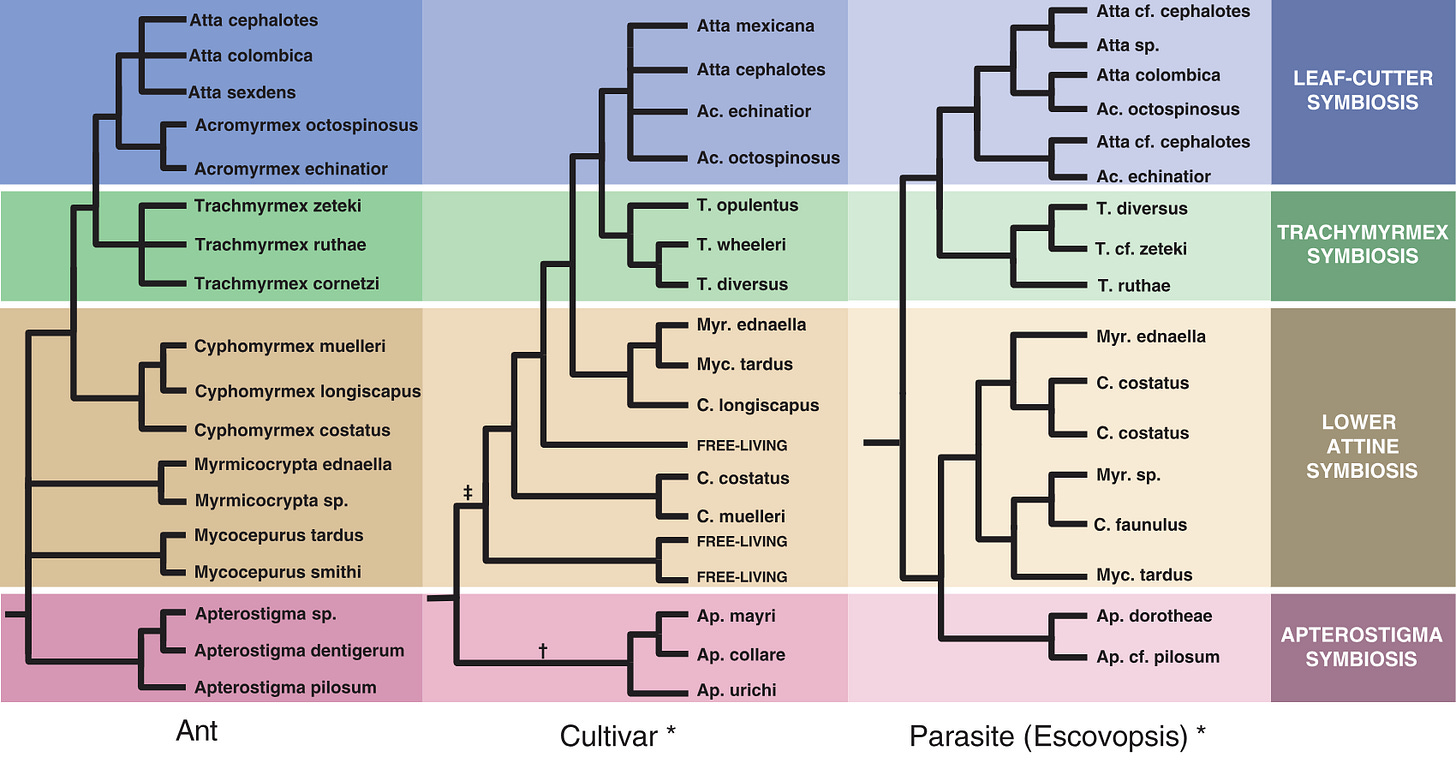
With genomic testing, scientists have discovered the extent of coevolution, such that you can see the same patterns in all four species: the ants, fungus, microfungus parasite, and bacteria are all evolving together, in complex, branching adaptations. You can clearly see striking similarities in the similar evolutionary trajectory of the ant (left), fungus (middle), and parasite (right):
Sometimes, though, parasites decisively win the coevolutionary battle. In ants, the most striking example is a fungus that turns ants into zombies. The fungus literally controls the ant’s behavior, taking over its mind, and marching it to a suitable location—typically on the northern side of a plant, 26 centimeters above the forest floor, in an environment with 94-95% humidity. Then, at precisely solar noon, the fungus makes the ant chomp down hard on the leaf, at which point lockjaw sets in, and the ant cannot release its death grip. At this point, the fungus kills the ant, then sprouts out of its head.
Similarly, cuckoo birds have engaged in a form of coevolution called “brood parasitism,” in which they lay eggs that mimic those of a variety of host species. Their eggs are thicker than the host eggs, so they’re protected from being broken; when the chicks hatch, they toss the other eggs out of the nest, to get the host bird to raise the cuckoo as though it’s its own. The host bird species have evolved ever-more elaborate markings on their eggs to try to differentiate their eggs from the parasitic bird eggs.
While this story is well-known, what’s astonishing—and has perplexed scientists until literally last week—is how cuckoos are able to produce eggs that mimic a variety of different species. Just look at the array below, with the cuckoo eggs on the top row and the host bird species eggs on the bottom. This is a coevolutionary arms race on vivid display:
The adaptations forged by coevolution are so tight-knit that one can even infer the existence of extinct species. Some species that live today coevolved with another species that no longer exists; the survivors that persist are called evolutionary anachronisms, or evolutionary ghosts, because they evolved in tandem with something that later disappeared.11
To find an evolutionary ghost, you need only look to the avocado. The avocado seed is ridiculously large—and there’s nothing alive today in their natural environment that could easily spread them. They evolved in an era of much larger megafauna, such as giant ground sloths, who would easily disperse the avocado seeds. Back then, the pit size was perfect; now it’s a hindrance to survival. (However, avocados continue to survive and thrive because humans now do the seed dispersal instead of the sloths.)
Next time you slice the pit from an avocado, contemplate how it likely evolved to be eaten whole and spread by now-extinct giant ground sloths.
IV: Biological Coevolution in Human Society
The science of coevolution also provides a powerful framework for better understanding humanity and complex social systems. Sometimes, the coevolution is literal—in which we coevolved with other species. Other times, coevolutionary principles offer a useful analytical framework for understanding how institutions emerge, how religions thrive or die out, and why arms races or parasitic relationships derail or supercharge human progress.
Dog-human coevolution is the most obvious form of biological adaptations between us and another species. As I previously wrote:
Researchers aren’t certain how domestication happened, but the prevailing theory is that starving wolves during an Ice Age realized that they could survive off of human food scraps. Those who were more docile were able to get closer to human settlements, eat more food, and therefore pass on their genes more effectively to the next generation. Humans, in turn, realized that having a wolf-like companion around was useful for hunting or guarding campsites.
A decade ago, researchers found that there were actual biological changes happening in that relationship that went far beyond selective breeding in dogs. For example, human bonding often occurs through eye contact, which releases oxytocin, a neurotransmitter which is sometimes called “the love hormone.”
Scientists documented that when humans and dogs make eye contact, it releases oxytocin for both species. Human-like social interactions create that biologically-mediated bond, something that is alien with other species. Indeed, when they tested wolves, no such eye contact or oxytocin relationship existed, even though dogs are descended from wolves.
Beyond our canine companions, as scientific research continues to reveal the astonishing complexity of our microbiome (there are roughly 1.3 bacterial cells inside us for every human cell, or, as Merlin Sheldrake once put it, there are “more bacteria in your gut than stars in our galaxy”), it’s becoming clear that our digestive and immune systems coevolved with many other species. And some of those species, which might even be helpful to us, are parasitic worms—helminths—even though our modern medical approach has always been to try to kill them off.
In 1970, Peter J. Preston, a doctor with the Royal Navy, noticed something peculiar: a dozen officers who got infected with human roundworm reported that their allergies had disappeared. This gave rise to decades of research which seems to suggest that some helpful species of worms can eliminate or alleviate a series of digestive symptoms, autoimmune disorders, and allergies. But what’s particularly striking is some recent research that seems to suggest infection with helpful gut worms is also potentially associated with decreased mental health disorders.
As William Parker of Duke University notes:
In collaboration with Staci Bilbo, a renowned neuroscientist at Duke University in North Carolina, we gave benign (harmless) helminths to female rats before the rats became pregnant. Surprising to some but anticipated by us, we found that the brains of baby rats (pups) are protected from inflammation if their mother has an intestinal worm. Thus, it seems likely that a complete loss of intestinal worms has something to do with the high rates of mental-health disorders in our children.
We are not rats, but the link is clearly worth exploring. However, before you infect yourself with worms, keep in mind that many species of worms are harmful parasites. It’s nonetheless likely that some species, which lived happily in most human guts until very recently, provided a function that was reciprocal, a form of mutualistic coevolution we’ve now disrupted.
V: Using Coevolution to Understand Social Change
When we write historical narratives, we focus on ourselves as the sole authors of our destinies. However, our societies often change in far more complex ways—and coevolution offers a compelling framework for much of our social development across the grand sweep of the human story.
I’ve previously highlighted how crucial genetic mutations that altered our shoulders from those of chimpanzees allowed us to uniquely throw objects accurately—which changed social dynamics so much that it is key to understanding the evolution of human social hierarchies in the ancient past.
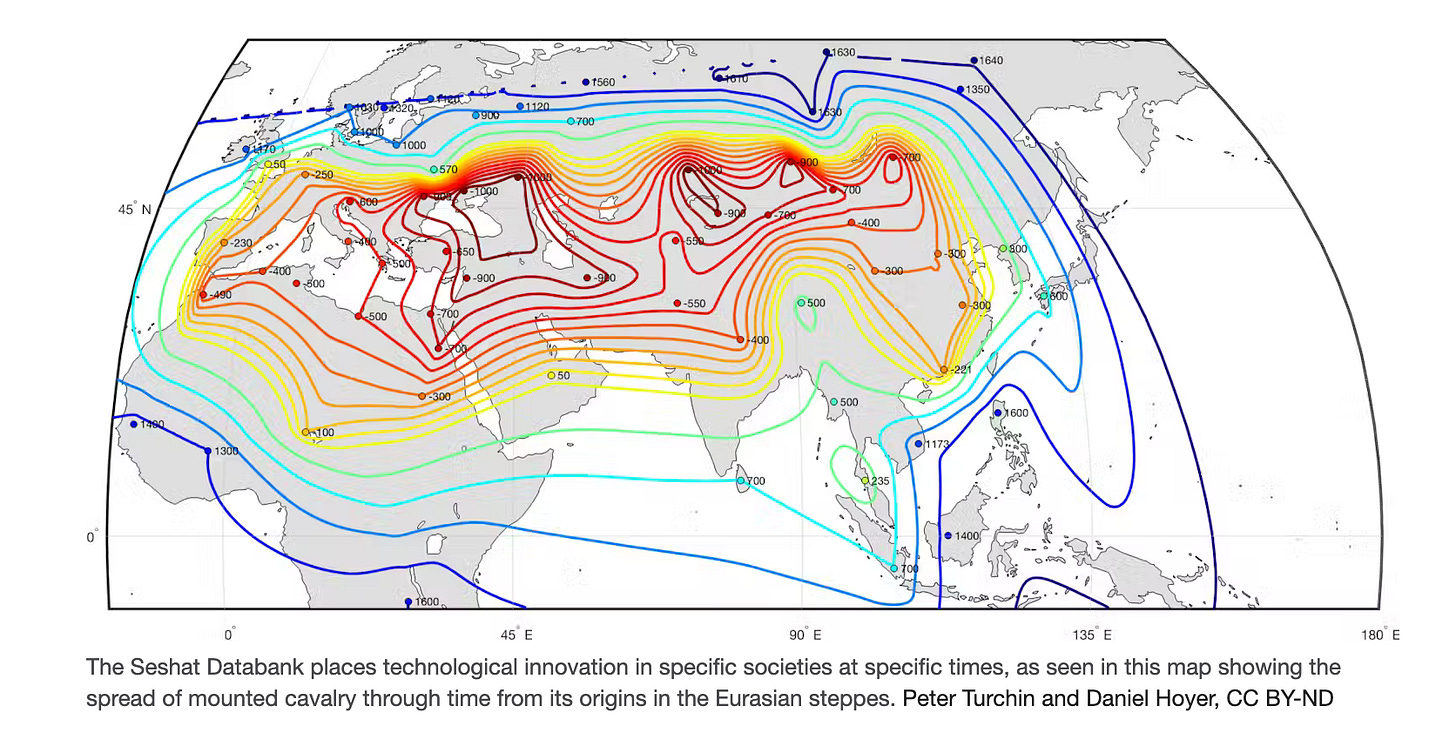
But recent research from Peter Turchin and his colleagues has also demonstrated how the combination of another species (horses) and human technological innovation (bits and bridles) was central the rise of complex civilization—and the origin of modern human society.
Their evaluation of Seshat (a comprehensive new historical database that aims to make historical claims more rigorously testable) has shown convincing evidence that the rise of sprawling mega-empires is typically preceded by the ability to ride horses and create a horse-human hybrid in the form of mounted cavalry. Their map shows the historic spread of that form of military technology, made possible by a symbiotic relationship with horses:
This was made possible by an evolutionary fluke, as Turchin notes:
It turns out that there were two mutations that enabled humans to ride horses. The first one (ZFPM1) made horses less mean, while the second one (GSDMC) enabled them to better bear weight. Both gene variants were very rare at the beginning of the third millennium BCE, but became common by the end of the millennium. Clearly, there was very strong (artificial) selection that drove this genetic shift.
These two mutations, and especially ZFPM1, which yielded tame horses, thus, were a historical accident with huge consequences for human history. It would not be much exaggeration to say that it was thanks to ZFPM1 that mega-empires and mega-religions appeared during the Axial Age. A closely related species, the zebra, has no comparable mutation and countless attempts to domesticate them all failed.
But beyond our relationships with other species, our societies also involve dynamics that are similar to biological coevolution, with repeated interaction, variation and experimentation, selection pressures, evolutionary niches with hotspots and coldspots, and individual behavior coevolving with changing systems. Many contemporary social science approaches ignore the coevolutionary framework, instead focusing too much on linear regressions. Our world is better understood through complex reciprocal interactions.
For example, as societies became more complex in the Axial Age, those civilizations needed a mechanism to create pro-social cooperation. In a comparative blink of human history, a series of ideas emerged—separated by huge distances—that made large-scale cooperation more possible, whether it was ever-watchful Big Gods who punished misdeeds or the sophisticated articulation of karma, rewarding proper behavior with an inescapable moral ledger. This implies a coevolutionary feedback loop: social structures swayed religious evolution, while religious norms unleashed cooperation and stability.
All around us today, we struggle with social problems that are also best understood through coevolution. Cybercrime battles between white hat and black hat hackers and the resurgence of nuclear weapons arms races are both clear examples of Red Queen-style stalemates. And the vapid postings of social media influencers are dictated by the evolutionary niches created by Silicon Valley algorithms, as engagement farming determines which behaviors are rewarded and which will die-off. Like Darwin’s Orchid, the YouTube moths morph to fit themselves perfectly into the algorithm’s nectary.
A hundred and sixty three years ago, a simple box containing beautiful flowers arrived at a house in southern England. In a single, memorable line of marveling at an unusual nectar tube, one of the greatest minds in human history provided us with a simple, powerful framework for understanding life—and ourselves: the stranger-than-fiction scientific magic of coevolution.
Thank you for reading The Garden of Forking Paths. If you value my research and writing (which, as you might have surmised while reading, takes a fair bit of work), then please consider upgrading to a paid subscription. It’s just $50/year, which works out to $4/month. Your generous support makes this possible—and keeps me writing for you. Alternatively, you can support my work by buying a copy of FLUKE.
The initial letter was written by Bateman’s son. Bateman then apologized for failing to include the labels, writing—in charming Victorian prose—that he “was very glad . . . that the orchids were so acceptable” but implored Darwin to overlook his oversight: “Pray forgive my gardener’s carelessness in omitting the names; when I charged him with the misdemeanor he defended himself on the ground that he could never have supposed you could have been ignorant of them!’ This scholarly article is the definitive account of Darwin’s Orchid and makes excellent reading even for a non-specialist.
Darwin did not always love orchids. On one occasion, he was fed up with them and wrote: “But I am very poorly today & very stupid & hate everybody & everything. One lives only to make blunders.- I am going to write a little Book for Murray on orchids & today I hate them worse than everything so farewell.” (This may be one of the most relatable scientific quotes ever written.)
The letter also speaks about the American Civil War and Darwin’s musings on how maybe it’s better if the south and north split for the sake of peace—but that he doesn’t want the slaveholders to win. Also, this is what J.D. Hooker’s beard looked like, which I am duty bound to share with you, dear reader, as it is magnificent:
The best scholarly article on the topic looked for written ridicule and didn’t find it, suggesting that Darwin may have been teased in person rather than in print. However, Alfred Russel Wallace, arguably the best field biologist of all-time, endorsed Darwin’s theory, writing that coevolution is as ironclad as natural laws of the solar system: “That such a moth exists in Madagascar may be safely predicted; and naturalists who visit that island should search for it with as much confidence as astronomers searched for the planet Neptune,—and they will be equally successful!”
For an interesting primer on coevolution, watch this lecture by Stephen Stearns.
If you’re interested in the hotspots/coldspots aspect, look up the Geographic Mosaic model of coevolution.
Several current biologists have contested this claim with more recent evidence.
Fungi are heterotrophs (as are we) meaning they can’t produce their own food through photosynthesis but instead obtain nutrients from the environment. Many thanks to Zack Blount, of Michigan State University, for pointing me to the research on the ant/fungus/parasite/bacteria phylogenetic relationship.
Examples include erythromycin, streptomycin, tetracycline, and vancomycin.
Another great example of an evolutionary ghost is the American pronghorn. It evolved alongside super rapid predators, such as the now-extinct American cheetah, so it’s believed that the pronghorn’s speed is a holdover from escaping those predators (now, it’s overkill, as it’s way faster than any possible predator).









thank you Brian for a gripping read. To my mind it prompted the thought that one could apply this coevolutionary framework to the emerging relationship between humans and AI. Which suggests exploring the idea of AI as a species. Perhaps that’s happening already under our noses as it were.
Thank you for another fascinating article. As I have said before, you always make me think, & learn new facts. 🤩